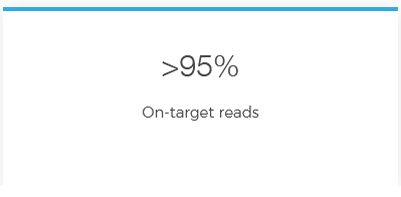
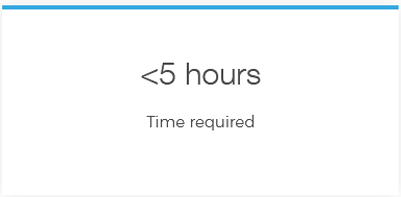
Detect all mutations in one test with Devyser’s CE-IVD single tube, next-generation sequencing (NGS) library prep kit for targeted, complete CFTR gene sequencing.
Discover the advantages of Devyser’s NGS workflow
 One tube per sample means no need for sample splitting
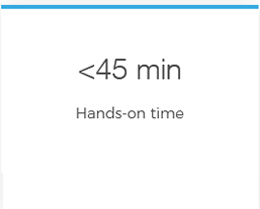
One tube per sample means no need for sample splitting Reduce hands-on time from days to under 45 minutes
Reduce hands-on time from days to under 45 minutes Direct detection of CNVs
Direct detection of CNVs Detect all mutations in the coding regions of the CFTR gene
Detect all mutations in the coding regions of the CFTR gene Determination of poly-T and TG repeats
Determination of poly-T and TG repeats Choice of several validated software options, including CNV analysis
Choice of several validated software options, including CNV analysis
Cystic Fibrosis Molecular Diagnostics
CFTR mutation testing can be used in diagnosis of cystic fibrosis, male infertility caused by CBAVD, and acute recurrent or chronic pancreatitis. It can also be used to guide targeted therapies, and as an aid in newborn screening. More than 2,000 mutations and variants in the CFTR gene have been described. The vast majority of these mutations have a population frequency below 0.1 % with high heterogeneity of mutation distribution between different ethnic groups. Devyser’s range of diagnostic CFTR kits enables allele specific detection as well as targeted, complete, CFTR gene sequencing using NGS for detection of all mutations, known and unknown, in a single test.
Designed for routine NGS diagnostics
The Devyser CFTR kit is easy to implement and highly cost-effective, making it a good match for laboratories of any size. With ready-to-use reagents and a user-friendly workflow, it suites both manual and automated workflows. Devyser’s unique single-tube approach simplifies the workflow, reduces hands-on time and minimizes the risk of sample mix-up and contamination. The proprietary multiplex PCR primer chemistry provides full and uniform coverage of the CFTR gene.